This section is designed to introduce a novel approach to performing measurements at chemical equilibrium in the Flow Injection format, with goal to facilitate optimization of reagent based assays.
Let us consider optimization of a spectrophotometric assay aimed at obtaining maximum sensitivity. Obviously, knowledge of molar absorptivity (ε) of the target species, will provide limit, which can not be exceeded by optimization of a assay protocol. Although (ε) values for reagent based assays are available in literature, it would be beneficial if (ε) value could be determined and verified by using the same instrument on which optimization of the optimized protocol will be performed. In other words, because (ε) values are determined by measurement performed at chemical equilibrium, they will serve as guide when influence of incubation time, temperature and reagent composition are investigated. Knowledge of (ε) value will also allow to calculate efficiency of preconcentration of target analyte (1.3.14.A.)
1.5.0.
Programmable Flow Injection as a Tool for
Research (and Teaching?)
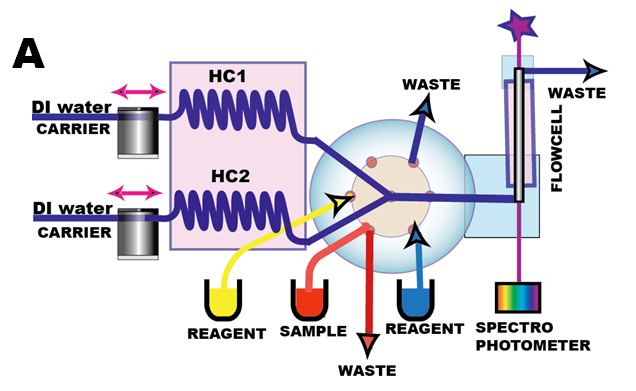

In the past, numerous configurations of flow injection system equipped with a stirred chamber have been designed (batch-flow systems), with aim to perform measurements on homogenous mixture and at equilibrium (Hansenís Database). However flow injection instrument configured without a mixing chamber will, have advantage of small internal volume, thus performing measurements in microfluidic format. The miniSIA-2 (A, B) instrument equipped with thermostated holding coils and 20 cm long flow can be programmed to perform in batch mode.
Feasibility of performing equilibrium measurements in batch format by pFI is documented in next sections on determination of (ε) values of bromothymol blue (BTB) and Fe(II) ferrozine complex as well as on determination of pKa of bromothymol blue.
Determination of pKa of BTB is a laboratory exercise frequently featured in undergraduate courses. Reported pKa values range from 7.0 to 7.5, probably due to differences in ionic strength of buffers, and other variables. Perhaps, some time in future, the labor intensive manual handling of multiple solutions and glassware will be replaced by automated computer controlled microfluidic manipulations in pFI format.









